Revefenacin Impurities
Revefenacin Impurity 19
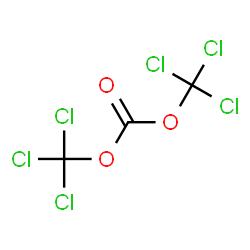
| CAT Number | CHR-9122018-4476 |
|---|---|
| CAS Number | 32315-10-9 |
| Mol.F | C3Cl6O3 |
| Mol.Wt | 296.748 g/mol |
| Inventory status | In-Stock |
Revefenacin is a medication for the treatment of chronic obstructive pulmonary disease. It was approved for use in the United States in 2018. It was developed by Theravance Biopharma and is marketed by Mylan. Revefenacin is formulated as a solution that is nebulized and inhaled.

| CAT Number | CHR-9122018-4476 |
|---|---|
| CAS Number | 32315-10-9 |
| Mol.F | C3Cl6O3 |
| Mol.Wt | 296.748 g/mol |
| Inventory status | In-Stock |